Phone:
(701)814-6992
Physical address:
6296 Donnelly Plaza
Ratkeville, Bahamas.
When seeking efficient, safe, and economical energy storage solutions, Sodium-ion (SIBs) and Lithium Iron Phosphate (LiFePO4) batteries have emerged as two technologies drawing significant attention. While both operate on the principle of ion movement, they exhibit notable differences in performance and application. This article will delve into the key parameters of these battery technologies, compare their strengths and limitations, and reveal which battery type is more suitable for specific use cases. As technology advances, both types of batteries are rapidly evolving, potentially offering more possibilities for sustainable energy storage in the future.
Sodium-ion battery (SIBs, NIBs, or Na ion battery) is a type of rechargeable battery that primarily relies on the movement of sodium ions between the cathode and anode for its operation, similar to the working principle of lithium-ion batteries. During charging, sodium ions (Na+) ions are deintercalated from the cathode and move to the anode; during discharging, Na+ ions return to the cathode from the anode, and electrons in the external circuit move from the anode to the cathode, reducing Na+ back to Na. Like lithium-ion batteries, sodium-ion batteries are also a type of rechargeable secondary battery.
Learn more: What is Sodium ion Battery Cell? LiFePO4 Alternative?

LiFePO4, also known as lithium iron phosphate, is a type of lithium-ion battery that has gained popularity for its safety, long lifespan, and stable performance. Unlike other lithium-ion batteries that may use cobalt or nickel in their cathodes, LiFePO4 batteries are composed of iron phosphate, which is more abundant and less toxic. This composition contributes to their lower risk of thermal runaway, a safety feature that is highly valued in applications such as electric vehicles and energy storage systems.
Here are some of the main criteria to consider when comparing the two types of solar batteries.
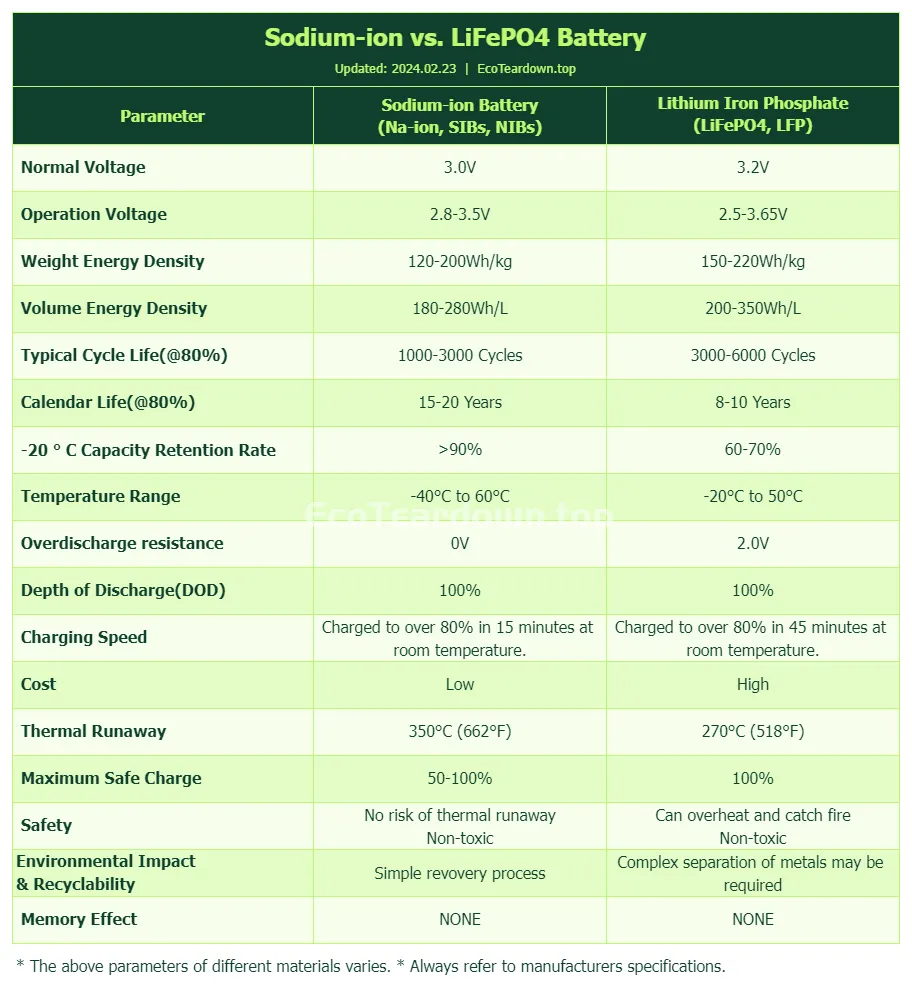
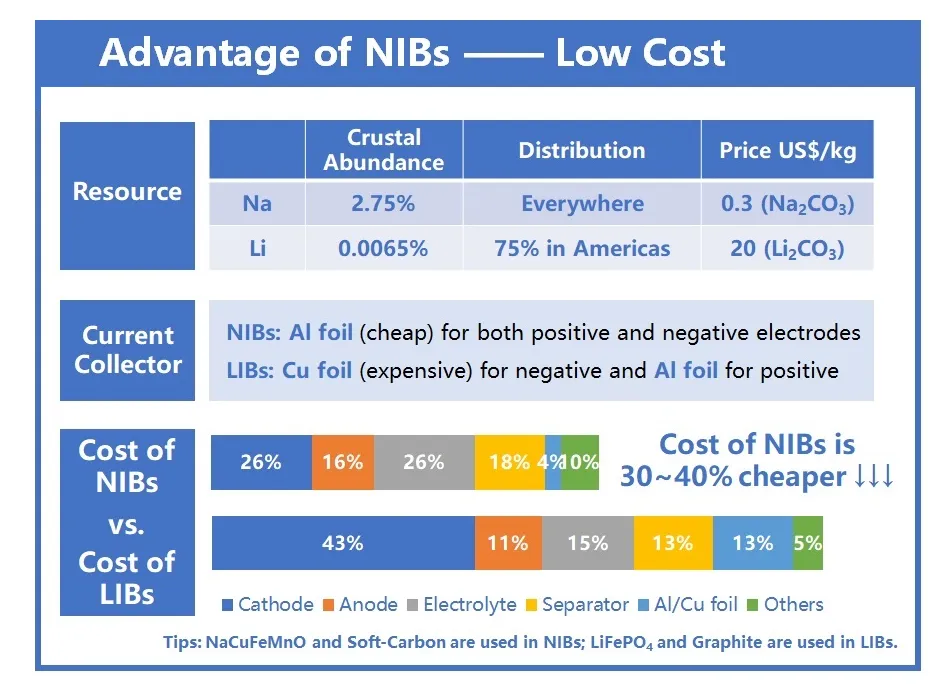
Sodium ion batteries are generally less expensive to produce due to the abundance and lower cost of sodium compared to lithium. This makes them an attractive option for large-scale energy storage and applications where cost is a significant factor.
LiFePO4 batteries typically offer a higher energy density than sodium ion batteries, meaning they can store more energy per unit volume or weight. This is advantageous for applications where space is limited or where high energy storage is required.
While sodium ion batteries currently have a lower energy density compared to lithium iron phosphate (LiFePO4) batteries, they are rapidly closing the gap. With ongoing research and development, it is expected that within the next two years, sodium ion batteries will achieve energy densities of 160-180Wh/kg, nearing the levels of LiFePO4 batteries. Projections suggest that within five years, sodium ion batteries could reach energy densities of around 200Wh/kg, further enhancing their cost-effectiveness.
Both types of batteries can achieve deep discharges, but LiFePO4 batteries often have a slight edge in this regard, allowing for a more complete discharge without significant impact on battery life.
LiFePO4 batteries are known for their high efficiency, with less energy lost during the conversion process from stored chemical energy to electrical energy. Sodium ion batteries are improving in this area but are generally less efficient.
LiFePO4 batteries have a longer lifespan with a higher number of cycles before performance significantly degrades. Sodium ion batteries are improving in this area, but they typically have a shorter cycle life compared to LiFePO4.
Both types of batteries can support fast charging, but the rate at which they can be charged without damaging the battery varies. LiFePO4 batteries often have a slight advantage in this area due to their stable chemistry.
Sodium ion batteries excel in cold conditions, a trait that traditional LiFePO4 batteries struggle with. This characteristic is particularly beneficial in regions with harsh winters, where the performance of LiFePO4 batteries can significantly degrade, leading to reduced range in electric vehicles. Sodium ion batteries’ ability to maintain their performance in low temperatures could be a game-changer for expanding the use of electric transportation in colder climates.
LiFePO4 batteries are considered to have a lower environmental impact due to the use of iron phosphate, which is more abundant and less toxic than other materials used in lithium-ion batteries. However, both types of batteries face challenges in recycling and disposal, which are areas of ongoing research and development.
Q: Can I replace a sodium ion battery with LiFePO4 battery?
A: It depends on the specific application and requirements. While both batteries have their advantages, they also have limitations that may make one more suitable than the other for a particular use case.
Q: What is the difference between sodium ion batteries and LiFePO4 batteries?
A: The main differences lie in cost, energy density, lifespan, efficiency, and performance under various conditions, as outlined above.
Q: What are the disadvantages of sodium ion batteries?
A: Sodium ion batteries generally have a lower energy density, shorter cycle life, and may be less efficient compared to LiFePO4 batteries. They also face challenges in recycling and disposal.
Both sodium ion and LiFePO4 batteries have their unique advantages and disadvantages, making them suitable for different applications. Sodium ion batteries offer a more cost-effective solution with better low-temperature performance, while LiFePO4 batteries provide higher energy density, longer lifespan, and improved safety features. The choice between the two will depend on the specific needs of the application, including cost, performance, and environmental considerations. As technology continues to advance, we can expect improvements in both types of batteries, potentially leading to more versatile and sustainable energy storage solutions.